http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0081821
Published: Nov 21, 2013
DOI: 10.1371/journal.pone.0081821
Abstract
Coral diseases are among the most serious threats to coral reefs worldwide, yet most coral diseases remain poorly understood. How the coral host responds to pathogen infection is an area where very little is known. Here we used next-generation RNA-sequencing (RNA-seq) to produce a transcriptome-wide profile of the immune response of the Staghorn coral Acropora cervicornis to White Band Disease (WBD) by comparing infected versus healthy (asymptomatic) coral tissues. The transcriptome of A. cervicornis was assembled de novo from A-tail selected Illumina mRNA-seq data from whole coral tissues, and parsed bioinformatically into coral and non-coral transcripts using existing Acropora genomes in order to identify putative coral transcripts. Differentially expressed transcripts were identified in the coral and non-coral datasets to identify genes that were up- and down-regulated due to disease infection. RNA-seq analyses indicate that infected corals exhibited significant changes in gene expression across 4% (1,805 out of 47,748 transcripts) of the coral transcriptome. The primary response to infection included transcripts involved in macrophage-mediated pathogen recognition and ROS production, two hallmarks of phagocytosis, as well as key mediators of apoptosis and calcium homeostasis. The strong up-regulation of the enzyme allene oxide synthase-lipoxygenase suggests a key role of the allene oxide pathway in coral immunity. Interestingly, none of the three primary innate immune pathways – Toll-like receptors (TLR), Complement, and prophenoloxydase pathways, were strongly associated with the response of A. cervicornis to infection. Five-hundred and fifty differentially expressed non-coral transcripts were classified as metazoan (n = 84), algal or plant (n = 52), fungi (n = 24) and protozoans (n = 13). None of the 52 putative Symbiodinium or algal transcript had any clear immune functions indicating that the immune response is driven by the coral host, and not its algal symbionts.
Figures
Citation: Libro S, Kaluziak ST, Vollmer SV (2013) RNA-seq Profiles of Immune Related Genes in the Staghorn Coral Acropora cervicornis Infected with White Band Disease. PLoS ONE 8(11): e81821. doi:10.1371/journal.pone.0081821
Editor: Kenneth Söderhäll, Uppsala University, Sweden
Received: August 22, 2013; Accepted: October 24, 2013; Published: November 21, 2013
Copyright: © 2013 Libro et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: Grant funding was provided by the NSF to Steve Vollmer (NSF-OCE 0751666). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The global rise in disease epidemics linked to climate change has taken a heavy toll on tropical reef-building corals and the diverse ecosystems they support [1-4]. A prime example is White Band Disease (WBD), which beginning in the late 1970s [5], caused unprecedented Caribbean-wide die-offs of two species of Acropora corals, the Staghorn coral A. cervicornis and the Elkhorn coral A. palmata [6-8]. As a result, both species are now listed as threatened on the US Endangered Species Act [9] and as critically endangered under the International Union for the Conservation of Nature (IUCN) Red List criteria [4]. Despite the devastating impacts of coral diseases on reefs world-wide, little is known about the basic etiology and ecology of most coral diseases [10-12] including basic information about how corals fight diseases [2,12,13], even though information about the coral immune response may be crucial to understanding the future resiliency of reef corals [2].
Genetic surveys indicate that corals and other cnidarians possess the genetic architecture underlying common innate immune pathways, including Toll-like receptors (TLR) as well as components of the complement and prophenoloxidase (PO) pathways [10,14,15]. PO activity and melanization responses have been elicited in corals exposed to pathogens [16-18] and components of the TLR pathway were differentially expressed in corals infected with non-host specific Symbiodinium types [19]. Elements of the complement pathway, such as mannose-binding lectins, appear to be involved in pathogen, symbiont, and self/nonself recognition in Acropora millepora [20]. Although cnidaria lack specialized immune cells, such as macrophages, cnidaria possess mobile amebocytes that are activated upon pathogen exposure or tissue damage [21-24]. Phagocytosis activity in cnidarians is commonly observed in flagellate gastrodermal cells during food uptake [25]. However, several studies have demonstrated that, upon immune stimulation, different populations of amebocytes can exhibit phagocitic activity directed toward wound healing and removal of necrotic tissue, as well as encapsulation of foreign particles [26,27].
Relatively few studies have studied the genetic response of corals infected with disease [28,29]. A microarray study of Pocillopora damicornis infected with Vibrio identified six candidate immune genes including three lectins and three putative antimicrobial proteins [28]. Exposure of A. millepora to bacterial and viral pathogen associated molecular patterns (PAMPs) resulted in up-regulation of few immune related genes including three GTPase of immunity associated proteins (GiMAP) [29], a family of conserved small GTPases involved in the antibacterial response of plants and mammals [30].
White Band Disease represents a good system to investigate the immune response of a reef-building coral. It is one of the few coral diseases that is highly transmissible [31] and host-specific [5,11]. WBD is characterized by an interface of white dying tissue that advances rapidly along the coral colony (Figure 1). Current evidence suggests that the pathogen is bacterial [31-36], but Henle-Koch postulates have not been satisfied. To date, multiple bacteria have been associated with WBD infections, including Vibrio harveyi [33,37] as well as a marine Rickettsia CAR1α [34]. In situ transmission experiments have identified naturally resistant and susceptible genotypes of A. cervicornis [31], indicating that the immune response to WBD varies among individuals.

Figure 1. White Band Disease on Acropora cervicornis.
A colony of the Staghorn coral A. cervicornis infected with White Band Disease showing the characteristic white band of dying and necrotic coral tissue.
doi:10.1371/journal.pone.0081821.g001
Here we used next-generation RNA-sequencing to produce a transcriptome-wide profile of the immune response of A. cervicornis to WBD by comparing infected versus healthy (asymptomatic) coral tissues. The transcriptome of A. cervicornis was assembled de novo from A-tail selected mRNA-seq data from whole coral tissues, and parsed bioinformatically into coral and non-coral transcripts using existing Acropora genomes in order to identify putative coral transcripts. Differentially expressed transcripts were identified in the coral and non-coral datasets to identify which genes were up- and down-regulated due to disease infection and characterize the immune response of the coral.
Results
A de novo assembly of the A. cervicornis transcriptome was assembled from 436.5 million Illumina RNA-sequencing reads from 45 coral samples of A. cervicornis and A. palmata. The total reads were de novo assembled using Trinity [38], resulting in 95,389 transcripts, with a N50 of 363 and N75 of 696. A total of 47,748 transcripts mapped against the existing Acropora genomes [39,40] and were classified as putative coral transcripts while the remaining 47,641 were classified as non-coral transcripts (Table 1).
Table 1. Summary of coral and non-coral transcripts.
Total number of transcripts (n), significantly differentially expressed transcripts (adj p-val<0.05) (DE), number of up-regulated (up) and down-regulated (down) transcripts among the entire dataset and annotated transcripts only (E-val<10-5). First row refers to putative coral transcripts, second row to non-coral transcripts.
For this study, five diseased (i.e. infected) and six healthy corals were used to profile the immune response of Staghorn corals infected with WBD. The average number of putative coral reads (±SE) was 4,076,829 (± 898,542) in the diseased coral samples compared to 4,199,946 (±761,894) in the healthy samples. In total, 20,503 coral transcripts (43 %) and 14,253 (30%) non-coral transcripts had strong protein annotations (Blastx e-value < 10-5) (Table 1).
Differentially expressed coral transcripts
Statistical analysis in DEseq [41] identified 1,805 differentially expressed (DE) transcripts (adj p-value < 0.05) between healthy and WBD coral samples (Table 1, Table S1); 559 of these DE transcripts had reliable protein annotations (Blastx e-values < 10-5) that could be used to characterize the immune response of A. cervicornis infected with WBD (Figure 2a, Figure 3). Annotated transcripts were characterized by gene ontology (GO) and grouped into manually curated categories based on literature searches highlighting immune functions (Table 2). WBD-infected corals exhibited strong gene expression responses for genes related to immunity (n = 72), apoptosis (n = 18) and arachidonic acid metabolism (n = 5). Calcification (n = 14) and calcium homeostasis (n = 21) were also perturbed, as well as cell growth and remodeling (n = 134), cellular processes (n = 188) and general metabolism (n = 43).
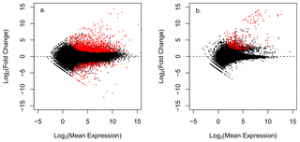
Figure 2. Volcano plots displaying differential gene expression between healthy and disease A. cervicornis.
Figure a. plots gene expression values of the putative coral transcripts, figure b. plots putative non coral transcripts. Each point represents an individual gene transcript. Red points represent significantly differentially expressed transcripts (adj p-value < 0.05).
doi:10.1371/journal.pone.0081821.g002

Figure 3. Heatmap of immune-related differentially expressed coral transcripts.
Table 2
Table 2. Summary of the main pathways involved in A.cervicornis response to WBD.
Number (N) of differentially expressed (DE) transcripts per category. Function defined by GO terms and manually curated categories. Expression values reported as log2fold change of WBD infected corals relative to healthy corals.
Immune-related processes
Sixty-nine DE transcripts were associated with immunity. Three C-type lectins receptors, C- type mannose receptor 2 (MRC2), macrophage lectin 2 (CLEC10A) and collectin-12 (COLEC12) were up-regulated in infected corals. Two mediators of phagocytosis were up-regulated – the macrophage receptor multiple epidermal growth factor-like domains protein 10 (MEGF10) and actin-22 (act22), which is involved in the phagosome formation. All three subunits of NADPH oxidase (NOX) involved in reactive oxygen species (ROS) production were up-regulated, including cytochrome b-245 heavy chain (CYBB), NADPH oxidase 3 (NOX3) and neutrophil cytosol factor 2 (p67-phox). Other DE immune related genes included nine antioxidants participating in the detoxification of ROS such as peroxidasin (PXDN, n=3) and glutaredoxin (GLRX), and 12 transcripts associated to response to stress such as golgi-associated plant pathogenesis-related protein 1 (GAPR-1, n = 3) and universal stress protein A-like protein (UspA, n = 2).
Little or no differential expression was detected in the three primary innate immune pathways – Toll/TLR, complement and prophenoloxidase (PO) pathways. In the Toll/TLR pathway, two TLR2 homologs and the adaptor molecule TNF receptor-associated factor 3 (TRAF3) were up-regulated in WBD corals. In the complement pathway, two transcripts encoding macrophage-expressed gene protein 1 (MPEG1) were differentially expressed, but they were down regulated in WBD corals. No differentially expressed transcripts were detected in the PO pathway.
Arachidonic acid metabolism
Six DE transcripts participating to the metabolism of arachidonic acid (AA) were up-regulated in diseased corals. Five matched coral allene oxide synthase-lipoxygenase (AOSL), a catalase related hemoprotein that catalyzes the biosynthesis of allene oxide, a precursor of marine eicoesanoids. The sixth transcript matched the enzyme phospholipase A2 (PLA2), involved formation of AA from membrane phospholipids.
Apoptosis
Eighteen DE transcripts were associated with apoptosis, including both pro- and anti-apoptotic regulators such as the extracellular matrix protein thrombospondin 2 and fibroblast growth factor receptor 2 (n = 2), respectively. Tumor necrosis factor receptor superfamily member 1A (TNFRSF1A) and caspase 3 (CASP-3) were up-regulated while caspase 8 (CASP-8) was down-regulated in WBD corals.
Calcification and calcium homeostasis
DE transcripts in this category included 14 proteins participating to carbon dioxide transport, biomineralization and skeletal growth. Two carbonic anhydrases were up-regulated (CA2 and CA3) and one was down-regulated (CA2) in WBD corals. Mediators of calcium homeostasis included 27 DE transcripts participating in calcium ion binding and transport such as calmodulin (CaM, n = 3), calumenin (CALU) and calsequestrin-2 (CASQ2) and were all up-regulated.
Cell growth and remodeling
Among the 138 DE transcripts related to cell growth and remodeling we identified 17 metallopeptidases (15 up, 2 down), 29 cytoskeletal proteins (all up-regulated) and 14 angiogenesis mediators (11 up, 3 down). A large group of DE transcripts were cell adhesion proteins (n = 29), including four up-regulated transcripts encoding sushi, von Willebrand factor type A, EGF and pentraxin domain-containing protein 1 (polydom/SVEP1).
Cell metabolism
Forty-two DE transcripts were associated with cell metabolism. These included 14 mediators of lipid metabolism, in particular, five lipases involved in lipid and phospholipid catabolism (n = 5, all up), such as pancreatic triacylglycerol lipase (PL), pancreatic lipase-related protein 2 (PL-RP2) and phospholipase DDHD1 (DDHD1). Four transcripts participating in fatty acid biosynthesis, such as fatty acid synthase (FASN), acetyl-CoA carboxylase (ACC) and acetyl-CoA carboxylase 1 (ACC1), were all down-regulated in WBD corals, and five transcripts involved in the breakdown of fatty acids such as long-chain-fatty-acid–CoA ligase 1 (LACS1) and 5 (LACS5) were up-regulated.
Non-coral transcripts
Out of the 47,641 putative non-coral transcripts in the dataset, 550 were differentially expressed in WBD infected corals (Table 1, Table S2). Of these 550 DE transcripts, 251 were well-annotated and were all up-regulated (Figure 2b). About 33 % were metazoan, the remaining were putative zooxanthellae (23%), fungi (10%) and protozoa (5%). A small number of transcripts matched bacteria (4%) and viruses (0.1%), while the remaining 23 % were unknown.
Metazoan transcripts (n = 84) included mediators of cell growth and remodeling (n = 16), metabolism (n = 4), cellular processes (n = 61) and two uncharacterized transcripts. Only two immune-related transcripts were identified and were the antioxidant peroxiredoxin-2 (PRDX2) and the metallopeptidase aminopeptidase O (AP-O), which may be involved in leukotrienes synthesis from AA.
Fifty-nine transcripts had plant, algae or Alveolata protein IDs and are presumed or putative Symbiodinum transcripts. Based on GO terms, these Symbiodinium transcripts were associated with cell growth and remodeling (n = 8), cellular processes (n= 38) and metabolism (n = 10), while two were uncharacterized. One transcript matched cysteine proteinase RD21a (RD21), a peptidase involved in defense against fungi. Fungal transcripts (n = 24) belonged to cell processes (n = 20) and metabolism (n = 3) plus one uncharacterized protein. Out of the 14 transcripts matching protozoa, 13 were associated to cellular processes, two to metabolism and one to cell growth and remodeling.
Nine transcripts matched bacterial proteins, six of them were involved in cellular processes (n = 3), metabolism (n = 3) and three were uncharacterized. Two transcripts shared protein IDs annotating to virus proteins (glycoprotein gp2 and one uncharacterized), while the remaining 59 transcripts did not have functional annotations.
Discussion
Our study demonstrates that Acropora cervicornis mounts a vigorous immune response against White Band Disease (WBD) pathogen(s) involving dramatic changes in gene expression across 4% of the coral transcriptome. The identities of the differentially expressed (DE) coral transcripts indicate that the response of A. cervicornis to WBD infection is driven by phagocytosis of apoptotic cells (Figure 3, Table 2). Corals infected with WBD exhibited strong differential expression of transcripts involved in macrophage-mediated pathogen recognition and ROS production, two hallmarks of phagocytosis, as well as key mediators of apoptosis and calcium homeostasis. The strong up-regulation of transcripts involved in arachidonic acid (AA) metabolism and allene oxide synthesis suggests their key role in coral immunity.
The primary signature of phagocytosis activity in WBD infected corals was the up-regulation of four macrophage receptors that recognize and bind to conserved motifs on the surface of target cells. Three of these receptors, MRC2, CLEC10A and COLEC12 belong to the C-type lectin family of proteins that include several Pathogen Recognition Receptors (PRRs). MRC2 recognizes mannose and fucose on glycoproteins of bacteria, viruses and fungi [42] while CLEC10A recognizes galactose and N-acetyl-galactosamine residues [43]. COLEC12is a scavenger receptor that shares structural similarity with macrophage scavenger receptor class A type I (SR-AI), a surface membrane receptor that mediates binding and phagocytosis of gram-positive, gram-negative bacteria and yeasts [44]. The fourth receptor, MEGF10, is membrane protein that promotes the clearance of apoptotic cells by causing macrophages to adhere and engulf them [45]. The stronger up-regulation of the three macrophage PRRs (2.2, 5.4 and 4.1 fold) compared to the one apoptotic cell recognizing receptor MEGF10 (2.17 fold) suggests the response is primarily driven by phagocytosis of microbes. A second signature of phagocytosis was the up-regulation of transcripts linked to ROS production, including three subunits of the enzymatic complex NADPH oxidase (NOX). ROS production is a general and highly conserved response to invading pathogens and stress and the release of ROS from the mitochondria can induce apoptosis in metazoan and yeasts [46,47]. During phagocytosis, ROS are generated in mature phagosomes (i.e. specialized vacuoles in phagocytic cells) [48] to kill engulfed cells [49]. In cnidarians, ROS production has been observed in the hydroid Hydra vulgaris exposed to the immune stimulant lipopolysaccaride (LPS) [50] and in reef corals during thermal and UV-induced bleaching [51,52], possibly due to the breakdown of the mitochondrial and photosynthetic membranes [53,54].
In WBD infected corals, it is possible that phagocytosis is aimed either at the removal of invading pathogens and/or used to clear damaged apoptotic cells [55]. The genetic signature of phagocytosis in WBD infected corals raises questions about the identity of these phagocytic immune cells in A. cervicornis. Cnidaria lack specialized immune cells, but do possess mobile amebocytes. Aggregations of amebocytes have been observed in the gorgonian coral Gorgonia ventalina infected with pathogenic fungi [24] and near wounded tissues in the soft coral Plexaurella fusifera [26]. Histological examination revealed that amebocytes exhibited phagocytic and PO activity [27] as well as antimicrobial activity against Gram-negative bacteria and ROS production [56]. Interestingly, certain populations of ameboid cells always show phagocytic activity, while others only acquire it upon immune activation [27]. These findings indicate that cnidaria, traditionally considered “simple” animals, are able to mount an innate immune response by employing the functional plasticity of amebocytes, which seem to represent the primary immune population of phagocytic cells.
Increased apoptosis in WBD infected corals was indicated by the differential expression of TNFRSF1A and CASP-3. During apoptosis, TNFRSF1A binds to tumor necrosis factor (TNF), which then recruits CASP-8 initiating the downstream activation of CASP-3, the main effector caspase of the apoptotic pathway [57,58]. While both TNFRSF1A and CASP-3 are up-regulated, CASP-8 is down-regulated which may suggest that CASP-3 is activated by some alternative pathway. Active programmed cell death was also suggested by disruption of calcium homeostasis as indicated by the strong up-regulation of CaM and other calcium binding proteins. In both plants and animals [59,60], apoptosis can be triggered by LPS from gram-negative bacteria via alteration of TNFRSF1A expression [61]. Some bacterial pathogens are also able to induce or inhibit apoptosis in their host [60,62,63] via alteration of membrane permeability and disruption of Ca2+ homeostasis [64], direct activation of TNF-α [65], TLR2 [66,67]or CASP-3 [68]. In corals, apoptosis occurs normally during metamorphosis [69] and the onset of symbiosis [70], but it has also been observed during bleaching as a possible mechanism to expel zooxanthellae in response to thermal stress [71-73]. Apoptosis has also been detected in the lesions of three Pacific species of Acropora infected by White Syndrome (WS), suggesting that it is a mechanism of tissue loss in WS [74].
Another key, yet unexpected, finding of this study is the potential role of the arachidonic acid (AA) pathway in the coral immune response. Genes involved in AA synthesis increased dramatically in WBD infected corals. The role of AA as an inflammation regulator is well-known in metazoans [75] , but has not been described in Cnidaria or in association with any coral disease. In metazoans, AA is released by apoptotic cells as chemotactic factor to promote clearance by phagocytes [76], but it can also induce apoptosis via rapid increase of calcium concentration and activation of CASP-3 in a CASP-8-independent way [77]. These findings are consistent with our data showing up-regulation of CASP-3, but not CASP-8, suggesting that AA may act similarly as immunomodulator in A. cervicornis. The five transcripts matching allene oxide synthase-lypoxigenases (AOSL) from the soft coral Plexaura homomalla, on the other hand, indicated that AA is converted into allene oxide, an intermediate compound of prostanoid synthesis in plants and soft corals [78-82].
Allene oxide has received considerable attention as a putative precursor of clavulones [83], a class of unique marine prostanoids known for their anti-viral and anti-cancer activity [84,85]. The link between the AOSL pathway and clavulones synthesis in corals, although still under debate, was suggested by the similarities with the biosynthetic pathway of jasmonic acid [83] a plant hormone that is produced via an allene oxide intermediate upon mechanical injury [86] and herbivore attack [87]. Although further study is needed to understand the role allene oxide in corals, our data represent the first evidence implicating AOSL in coral immunity and suggest that AOSL may be involved in controlling levels of free AA produced by apoptotic cells.
Several other immune related genes exhibited altered expression in infected corals. The majority were anti-oxidants including PXDN, peroxidasin-like proteins and GLRX – a glutathione-dependent enzyme. PXDN has been shown to be DE in some thermally stressed corals, but not in a consistent manner. For example, in Montastraea faveolata, PXDN was up-regulated in thermally-stressed larvae [88], but was down-regulated in thermally-bleached adult colonies [89]. Active cell remodeling and cell matrix degradation was indicated by several DE cytoskeletal proteins, metalloproteases and cell adhesion proteins, probably associated with cellular and cytoskeletal rearrangements linked to phagocytosis and apoptosis. CASP-3 activation, in particular, initiates apoptosis by altering the expression of metalloproteases and hydrolytic enzymes such as cathepsins that degrade extracellular matrix components [90]. Interestingly, WBD infected corals up-regulated three transcripts encoding polydom, a cell adhesion protein belonging to the pentraxin family of lectins. Recent studies suggest an immune function for polydom based on its similarities in its protein domains to complement proteins and C-type lectins with antimicrobial activity [91]. In cnidarians, the potential immune role for polydom is bolstered by its up-regulation in the hydroid Hydractinia symbiolongicarpus after fungal and bacterial exposure [92].
Surprisingly, none of the three main innate immune pathways – TLR, complement and PO – played a prominent role in the immune signature of A. cervicornis infected with WBD, even though transcripts from these pathways are well-represented in our transcriptome. Only three transcripts in the TLR pathway were differentially expressed: two TLRs matching to human TLR2 and TRAF3. In the lectin complement pathway, the only two DE transcripts were two proteins matching MPEG1, a MAC/PF (membrane attack complex/perforin) containing protein that is involved in the response against Gram negative bacteria in sponges and is up-regulated upon LPS exposure [93]. None of the transcripts belonging to the PO pathway were differentially expressed during WBD infection, even though in other corals PO activity acts as an important defense against invading pathogens and tissue damage [16-18].
Non-coral transcripts
The taxonomic distribution of non-coral transcripts highlighted the presence of several members of the coral holobiont, i.e. the coral host and associated symbiotic microorganisms, including zooxanthellae, fungi and protozoa. The majority of these non-coral transcripts matched metazoan and putative zooxanthaellae proteins, while the remaining transcripts matched fungi, protozoa and bacteria. GO term analysis revealed that most of these non-coral transcripts encoded mediators of cell homeostasis and general metabolism. Transcripts with metazoan identities were likely coral transcripts that did not have identities in the coral reference genomes and may thus represent transcripts unique to A. cervicornis. Putative zooxanthellae transcripts were identified as transcripts annotating to Viridiplantae, Heterokontophyta (i.e. algae), cyanobacteria and the superphylum Alveolata. Interestingly, no genetic signature of immune activity from the algal symbionts was evident in our transcriptome. Instead, our data suggest drastic changes in photosynthesis and cell metabolism of the zooxanthellae; this is consistent with a previous study showing that Symbiodinum undergo major alteration of carbon metabolism in response to stress [94].
Conclusions
Our data reveal that the coral host, but not its algal symbionts, undergoes dramatic alterations in gene expression during response to WBD infection. Transcriptional changes affected mediators of innate immunity, in particular receptors on the surface of phagocytic cells, enzymes involved in ROS production and modulators of apoptosis. Taken together, our data suggest that WBD infection in A. cervicornis is associated with apoptosis, and that WBD pathogen triggers a powerful immune response driven by phagocytic cells that encapsulate and degrade apoptotic cells. This study also indicates a key role for arachidonic acid and in particular the enzyme AOSL in A. cervicornis immunity.
Materials and Methods
Total RNA was extracted from diseased and healthy Acropora cervicornis sampled from Crawl Cay reef in Bocas del Toro, Panama under Autoridad Nacional del Ambiente (ANAM) Collecting permit SE/A-71-08. For the diseased samples, corals with active mobile WBD interfaces were identified by monitoring the mobility of disease interfaces for two days, and then sampling a 2 cm region of tissue at and above the disease interface. A comparably sized and located tissue sample was taken from healthy (i.e. asymptomatic) corals. The coral tissues were flash frozen in liquid nitrogen and stored at -80°C. Total RNA was extracted in TriReagent (Molecular Research Center, Inc.) following the manufacturer’s protocol. Total RNA quality was assessed using the RNA Pico Chips on an Agilent Bioanalyzer 2100, and only extractions showing distinctive 28S and 18S bands and RIN values of 6 or higher were prepped for RNA sequencing.
RNA sequencing was performed on five diseased and six healthy coral samples using a multiplexed Illumina mRNA-seq protocol [95] with the following modifications. Instead of fragmenting the mRNA prior to cDNA synthesis, we obtained much better success fragmenting the double stranded cDNA using DNA fragmentase (New England Biolabs) for 30 minutes at 37°C. RNA-seq libraries were then prepared using next-generation sequencing modules (New England Biolabs) and custom paired-end adapters with 4bp barcodes. Multiplexed samples were run (2-3 samples per lane) on the Illumina GAII platform (Illumina, Inc, San Diego, California, USA) at the FAS Center for System Biology at Harvard University. Barcoded samples were de-multiplexed and raw sequencing reads were quality trimmed to remove sequences and regions with a Phred score of less than 30 and a read length less than 15bp long using custom Perl Scripts in the FASTX-Toolkit (http://hannonlab. cshl.edu/fastx_toolkit/).
A de novo transcriptome was assembled using Trinity [38] from 463.5 million single-end Illumina RNA-Seq reads from 39 A. cervicornis and 6 A. palmata samples, including the 11 A. cervicornis samples included in this paper. The assembled transcriptome produced 95,389 transcripts with a N50 of 363 and N75 of 696. RNA-seq data were produced using whole coral tissue, which putatively contains sequences from the coral host, its algal symbiont Symbiodinium, and other members of the coral holobiont (e.g. fungi, bacteria, and viruses).
In order to resolve the holobiont, and putatively classify the source of the transcripts that were assembled as either coral or non-coral, we utilized a multistep pipeline leveraging the existing genomes of two congener species – A. digitifera [39] and A. millepora [40]. RNA-seq reads were mapped against both Acropora reference genomes using Bowtie [96] to produce two exomes. Transcripts from our de novo assembly were aligned using BLAST [97] against each exome. Transcripts were assigned as putatively coral if they matched either exome with an e-value of less than 10-10. Transcripts without significant coral hits were assigned as non-coral and could potentially include novel coral and/or algal symbiont Symbiodinium transcripts, as well as other associated eukaryotes, like endolithic fungi. Bacterial and viral transcripts are possible, but less likely given that A-tail selection to isolate eukaryotic mRNAs was performed prior to cDNA synthesis.
Putative gene identities for each transcript were identified by performing homology searches against the Swiss-Prot and TReMBLE protein databases [98], using tBLASTx. Matches with an e-value of less than10-5 were considered homologous protein-coding genes. Subsequently, GenBank Flat Files corresponding to the hits’ Accession ID’s were downloaded and used to extract taxonomic data for each used as a second method to identify the putative source of the transcripts. GO terms and gene functions were obtained for the annotated transcripts on UniProt. The reference transcriptome sequences are available on Bioproject (accession number PRJNA222758).
Differences in gene expression between healthy and disease A.cervicornis specimens were estimated using the R package DESeq [38]. First, all contigs were separated into two datasets –i.e. coral and non-coral- based on their matches to the Acropora genomes. Size factor estimation and normalization were then performed separately on each dataset using the functions estimateSizeFactors and estimateDispersions, respectively. Differentially expressed contigs were detected by running a negative binomial test using the function nbinomTest. Only differentially expressed transcripts (adjusted p-value < 0.05) that were also annotated (e-values < 10-5) were used for this study.
Supporting Information
Table_S1.xlsx
Table_S1
Table S2.
Dataset of annotated (E-val<10-5 ) non-coral transcripts exhibiting differential expression between healthy and diseased samples (adj p-val<0.05).
Table s2
Acknowledgments
The authors would like to thank Elizabeth Hemond for helping with sample collection and library preparation, Laura Geyer and the Smithsonian Tropical Research Institute and the for field and logistical support and the members of the Vollmer lab for valuable comments. Collection permits were provided by Autoridad Nacional del Ambiente (ANAM) SE/A-71-08.
Author Contributions
Conceived and designed the experiments: SV. Performed the experiments: SL. Analyzed the data: SL STK. Contributed reagents/materials/analysis tools: SV. Wrote the manuscript: SL SV.
References
1. Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR et al. (1999) Emerging marine diseases–climate links and anthropogenic factors. Science 285: 1505-1510. doi:. PubMed: 10498537. doi: 10.1126/science.285.5433.1505.
2. Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR et al. (2003) Climate Change, Human Impacts, and the Resilience of Coral Reefs. Science 301: 929-933. doi:. PubMed: 12920289. doi: 10.1126/science.1085046.
3. Harvell D, Aronson R, Baron N, Connell J, Dobson A et al. (2004) The rising tide of ocean diseases: unsolved problems and research priorities. Frontiers in Ecology and the Environment 2: 375-382. Available online at: doi:10.1890/1540-9295(2004)002[0375:TRTOOD]2.0.CO; 2.
4. Carpenter KE, Abrar M, Aeby G, Aronson RB, Banks S et al. (2008) One-Third of Reef-Building Corals Face Elevated Extinction Risk from Climate Change and Local Impacts. Science 321: 560-563. PubMed: 18653892. doi: 10.1126/science.1159196.
5. Gladfelter WB (1982) White-Band Disease in Acropora palmata – Implications for the structure and growth of shallow reefs. Bulletin of Marine Science 32: 639-643.
CrossRef
PubMed/NCBI
Google Scholar
6. Aronson RB, Precht WF (1997) Stasis, biological disturbance, and community structure of a Holocene coral reef. Paleobiology 23: 326-346.
CrossRef
PubMed/NCBI
Google Scholar
7. Aronson R, Precht W (2001) White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia 460: 25-38. doi:. doi: 10.1007/978-94-017-3284-0_2.
8. Aronson RB, Macintyre IG, Precht WF, Murdoch TJT, Wapnick CM (2002) The Expanding Scale of Species Turnover Events on Coral Reefs in Belize. Ecological Monographs 72: 233-249. Available online at: doi:10.1890/0012-9615(2002)072[0233:TESOST]2.0.CO; 2.
9. Hogarth WT (2005) Endangered and threatened species: proposed threatened status for elkhorn coral and staghorn coral. Federal Register 70: 24359-24365.
CrossRef
PubMed/NCBI
Google Scholar
10. Richardson LL, Goldberg WM, Kuta KG, Aronson RB, Smith GW et al. (1998) Florida’s mystery coral-killer identified. Nature 392: 557-558. doi:. doi: 10.1038/33302.
11. Sutherland KP, Porter JW, Torres C (2004) Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Marine Ecology Progress Series 266: 273-302. doi:. doi: 10.3354/meps266273.
12. Mydlarz LD, McGinty ES, Harvell CD (2010) What are the physiological and immunological responses of coral to climate warming and disease? J Exp Biol 213: 934-945. doi:. PubMed: 20190118. doi: 10.1242/jeb.037580.
13. Mydlarz LD, Jones LE, Harvell CD (2006) Innate Immunity, Environmental Drivers, and Disease Ecology of Marine and Freshwater Invertebrates. Annual Review of Ecology, Evolution, and Systematics 37: 251-288. doi:. doi: 10.1146/annurev.ecolsys.37.091305.110103.
14. Miller DJ, Hemmrich G, Ball EE, Hayward DC, Khalturin K et al. (2007) The innate immune repertoire in cnidaria–ancestral complexity and stochastic gene loss. Genome Biol 8: R59. doi:. PubMed: 17437634. doi: 10.1186/gb-2007-8-4-r59.
15. Dunn SR (2009) Immunorecognition and immunoreceptors in the Cnidaria. Invertebrate Surviv J 6: 7-14.
CrossRef
PubMed/NCBI
Google Scholar
16. Palmer CV, Mydlarz LD, Willis BL (2008) Evidence of an inflammatory-like response in non-normally pigmented tissues of two scleractinian corals. Proc Biol Sci 275: 2687-2693. doi:. PubMed: 18700208. doi: 10.1098/rspb.2008.0335.
17. Mydlarz LD, Couch CS, Weil E, Smith G, Harvell CD (2009) Immune defenses of healthy, bleached and diseased Montastraea faveolata during a natural bleaching event. Dis Aquat Organ 87: 67-78. doi:. PubMed: 20095242. doi: 10.3354/dao02088.
18. Palmer CV, Bythell JC, Willis BL (2010) Levels of immunity parameters underpin bleaching and disease susceptibility of reef corals. FASEB J 24: 1935-1946. doi:. PubMed: 20124432. doi: 10.1096/fj.09-152447.
19. Voolstra CR, Schwarz JA, Schnetzer J, Sunagawa S, Desalvo MK et al. (2009) The host transcriptome remains unaltered during the establishment of coral–algal symbioses. Mol Ecol 18: 1823-1833. doi:. PubMed: 19317843. doi: 10.1111/j.1365-294x.2009.04167.x.
20. Kvennefors EC, Leggat W, Hoegh-Guldberg O, Degnan BM, Barnes AC (2008) An ancient and variable mannose-binding lectin from the coral Acropora Millepora binds both pathogens and symbionts. Dev Comp Immunol 32: 1582-1592. doi:. PubMed: 18599120. doi: 10.1016/j.dci.2008.05.010.
21. Young JAC (1974) The Nature of Tissue Regeneration After Wounding in the Sea Anemone Calliactis Parasitica (Couch). Journal of the Marine Biological Association of the United Kingdom 54: 599-617. doi:. doi: 10.1017/s0025315400022773.
22. Patterson MJ, Landolt ML (1979) Cellular reaction to injury in the anthozoan Anthopleura elegantissima. Journal of Invertebrate Pathology 33: 189-196. doi:. doi: 10.1016/0022-2011(79)90152-6.
23. Ellner SP, Jones LE, Mydlarz LD, Harvell CD (2007) Within-Host Disease Ecology in the Sea Fan Gorgonia ventalina: Modeling the Spatial Immunodynamics of a Coral-Pathogen Interaction. Am Nat 170: E143-E161. doi:. PubMed: 18171161. doi: 10.1086/522841.
24. Mydlarz LD, Holthouse SF, Peters EC, Harvell CD (2008) Cellular Responses in Sea Fan Corals: Granular Amoebocytes React to Pathogen and Climate Stressors. PLOS ONE 3: e1811. doi:. PubMed: 18364996. doi: 10.1371/journal.pone.0001811.
25. Chapman D (1974) Cnidarian Histology. In Coelenterate Biology Reviews and New Perspectives L. MuscatineHM Lenhoff. Academic Press, New York. pp. 2–92.
26. Meszaros A, Bigger CH (1999) Qualitative and quantitative study of wound healing processes in the coelenterate, Plexaurella fusifera: spatial, temporal, and environmental (light attenuation) influences. J Invertebr Pathol 73: 321-331. doi:. PubMed: 10222188. doi: 10.1006/jipa.1999.4851.
27. Olano CT, Bigger CH (2000) Phagocytic activities of the gorgonian coral Swiftia exsertia. J Invertebr Pathol 76: 176-184. doi:. PubMed: 11023745. doi: 10.1006/jipa.2000.4974.
28. Vidal-Dupiol J, Ladrière O, Meistertzheim AL, Fouré L, Adjeroud M et al. (2011) Physiological responses of the scleractinian coral Pocillopora damicornis to bacterial stress from Vibrio coralliilyticus. J Exp Biol 214: 1533-1545. doi:. PubMed: 21490261. doi: 10.1242/jeb.053165.
29. Weiss Y, Forêt S, Hayward DC, Ainsworth T, King R et al. (2013) The acute transcriptional response of the coral Acropora Millepora to immune challenge: expression of GiMAP/IAN genes links the innate immune responses of corals with those of mammals and plants. BMC Genomics 14: 400. doi:. PubMed: 23768317. doi: 10.1186/1471-2164-14-400.
30. Wang Z, Li X (2009) IAN/GIMAPs are conserved and novel regulators in vertebrates and angiosperm plants. Plant Signaling & Behavior 4: 165-167. doi:. doi: 10.4161/psb.4.3.7722.
31. Vollmer SV, Kline DI (2008) Natural Disease Resistance in Threatened Staghorn Corals. PLOS ONE 3: e3718. PubMed: 19005565. doi: 10.1371/journal.pone.0003718.
32. Peters EC, Oprandy JJ, Yevich PP (1983) Possible causal agent of “white band disease” in caribbean acroporid corals. Journal of Invertebrate Pathology 41: 394. doi:. doi: 10.1016/0022-2011(83)90260-4.
33. Ritchie KB, Smith GW (1998) Type II white-band disease. Revista De Biologia Tropical 46: 199-203.
CrossRef
PubMed/NCBI
Google Scholar
34. Casas V, Kline DI, Wegley L, Yu Y, Breitbart M et al. (2004) Widespread association of a Rickettsiales-like bacterium with reef-building corals. Environ Microbiol 6: 1137-1148. doi:. PubMed: 15479247. doi: 10.1111/j.1462-2920.2004.00647.x.
35. Pantos O, Bythell JC (2006) Bacterial community structure associated with white band disease in the elkhorn coral Acropora palmata determined using culture-independent 16S rRNA techniques. Dis Aquat Organ 69: 79-88. doi:. PubMed: 16703769. doi: 10.3354/dao069079.
36. Gignoux-Wolfsohn SA, Marks CJ, Vollmer SV (2012) White Band Disease transmission in the threatened coral, Acropora cervicornis. Sci Rep 2: 804. PubMed: 23150775. doi: 10.1038/srep00804.
37. Gil-Agudelo DL, Smith GW, Weil E (2006) The white band disease type II pathogen in Puerto Rico. Revista De Biologia Tropical 54: 59-67. PubMed: 18457175.
38. Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA et al. (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29: 644-652. doi:. PubMed: 21572440. doi: 10.1038/nbt.1883.
39. Shinzato C, Shoguchi E, Kawashima T, Hamada M, Hisata K et al. (2011) Using the Acropora digitifera genome to understand coral responses to environmental change. Nature 476: 320-323. doi:. PubMed: 21785439. doi: 10.1038/nature10249.
40. Miller DJ (unpublished). Acropora Millepora genome. http://genome.wustl.edu/genomes/detail/acropora-millepora/. Available:
41. Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome Biol 11: R106. doi:. PubMed: 20979621. doi: 10.1186/gb-2010-11-10-r106.
42. Stahl PD, Ezekowitz RAB (1998) The mannose receptor is a pattern recognition receptor involved in host defense. Curr Opin Immunol 10: 50-55. doi:. PubMed: 9523111. doi: 10.1016/s0952-7915(98)80031-9.
43. Suzuki N, Yamamoto K, Toyoshima S, Osawa T, Irimura T (1999) Molecular cloning and expression of cDNA encoding human macrophage C-type lectin: its unique carbohydrate binding specificity for Tn antigen. J Immun 156: 128-135.
CrossRef
PubMed/NCBI
Google Scholar
44. Nakamura K, Funakoshi H, Miyamoto K, Tokunaga F, Nakamura T (2001) Molecular Cloning and Functional Characterization of a Human Scavenger Receptor with C-Type Lectin (SRCL), a Novel Member of a Scavenger Receptor Family. Biochemical and Biophysical Research Communications 280: 1028-1035. doi: 10.1006/bbrc.2000.4210.
CrossRef
PubMed/NCBI
Google Scholar
45. Suzuki E, Nakayama M (2007) The mammalian Ced-1 ortholog MEGF10/KIAA1780 displays a novel adhesion pattern. Experimental Cell Research 313: 2451-2464. doi: 10.1016/j.yexcr.2007.03.041.
CrossRef
PubMed/NCBI
Google Scholar
46. Madeo F, Fröhlich E, Ligr M, Grey M, Sigrist SJ et al. (1999) Oxygen Stress: A Regulator of Apoptosis in Yeast. J Cell Biol 145: 757-767. doi:. PubMed: 10330404. doi: 10.1083/jcb.145.4.757.
47. Simon HU, Haj-Yehia A, Levi-Schaffer F (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5: 415-418. doi:. PubMed: 11256882. doi: 10.1023/a:1009616228304.
48. Brown BE, Howard LS (1985) Assessing the effects of “stress” on reef corals. Advances in Marine Biology. London, Academic Press.
49. Imlay J, Linn S (1988) DNA damage and oxygen radical toxicity. Science 240: 1302-1309. doi: 10.1126/science.3287616.
CrossRef
PubMed/NCBI
Google Scholar
50. Zelensky AN, Gready JE (2003) Comparative analysis of structural properties of the C-type-lectin-like domain (CTLD). Proteins: Structure, Function, and Bioinformatics 52: 466-477. doi: 10.1002/prot.10626.
CrossRef
PubMed/NCBI
Google Scholar
51. Dykens JA, Shick JM, Benoit C, Buettner GR, Winston GW (1992) Oxygen Radical Production in the Sea Anemone Anthopleura Elegantissima and its Endosymbiotic Algae. Journal of Experimental Biology 168: 219-241.
CrossRef
PubMed/NCBI
Google Scholar
52. Nii CM, Muscatine L (1997) Oxidative Stress in the Symbiotic Sea Anemone Aiptasia pulchella (Carlgren, 1943): Contribution of the Animal to Superoxide Ion Production at Elevated Temperature. Biological Bulletin 192: 444-456. doi:. doi: 10.2307/1542753.
53. Brown MPS, Grundy WN, Lin D, Cristianini N, Sugnet CW et al. (2000) Knowledge-based analysis of microarray gene expression data by using support vector machines. Proceedings of the National Academy of Sciences of the USA 97: 262-267. doi:. PubMed: 10618406. doi: 10.1073/pnas.97.1.262.
54. Franklin DJ, Hoegh-Guldberg O, Jones RJ, Berges JA (2004) Cell death and degeneration in the symbiotic dinoflagellates of the coral Stylophora pistillata during bleaching. Marine Ecology Progress Series 272: 117-130. doi:. doi: 10.3354/meps272117.
55. Henson PM, Bratton DL, Fadok VA (2001) Apoptotic cell removal. Curr Biol 11: R795-R805. doi:. PubMed: 11591341. doi: 10.1016/s0960-9822(01)00474-2.
56. Hutton MC, Smith VJ (1996) Antibacterial properties of isolated amebocytes from the sea anemone Actinia equina. Biol Bull 191: 441-451. doi:. doi: 10.2307/1543017.
57. Duchen MR (2000) Mitochondria and calcium: from cell signalling to cell death. J Physiol 529: 57-68. doi:. PubMed: 11080251. doi: 10.1111/j.1469-7793.2000.00057.x.
58. Yu W, Niwa T, Miura Y, Horio F, Teradaira S et al. (2002) Calmodulin Overexpression Causes Ca2+-Dependent Apoptosis of Pancreatic [bgr] Cells, Which Can Be Prevented by Inhibition of Nitric Oxide Synthase. Lab Invest 82: 1229-1239. doi:. PubMed: 12218084. doi: 10.1097/01.LAB.0000027921.01548.C5.
59. Mittler R, Simon L, Lam E (1997) Pathogen-induced programmed cell death in tobacco. J Cell Sci 110: 1333-1344. PubMed: 9202394.
60. Gao L-Y, Kwaik YA (2000) The modulation of host cell apoptosis by intracellular bacterial pathogens. Trends Microbiol 8: 306-313. doi:. PubMed: 10878765. doi: 10.1016/s0966-842x(00)01784-4.
61. Alikhani M, Alikhani Z, Graves DT (2004) Apoptotic Effects of LPS on Fibroblasts are Indirectly Mediated through TNFR1. J Dent Res 83: 671-676. doi:. PubMed: 15329370. doi: 10.1177/154405910408300903.
62. Zychlinsky A, Prevost MC, Sansonetti PJ (1992) Shigella flexneri induces apoptosis in infected macrophages. Nature 358: 167-169. doi:. PubMed: 1614548. doi: 10.1038/358167a0.
63. Weinrauch Y, Zychlinsky A (1999) The induction of apoptosis by bacterial pathogens. Annu Rev Microbiol 53: 155-187. doi:. PubMed: 10547689. doi: 10.1146/annurev.micro.53.1.155.
64. Ayala P, Vasquez B, Wetzler L, So M (2002) Neisseria gonorrhoeae Porin P1.B Induces Endosome Exocytosis and a Redistribution of Lamp1 to the Plasma. Membr – Infection and Immunity 70: 5965-5971. doi:. doi: 10.1128/iai.70.11.5965-5971.2002.
65. Rojas M, Olivier M, Gros P, Barrera LF, García LF (1999) TNF-α and IL-10 Modulate the Induction of Apoptosis by Virulent Mycobacterium tuberculosis in Murine Macrophages. J Immunol 162: 6122-6131. PubMed: 10229855.
66. Aliprantis AO, Yang R-B, Mark MR, Suggett S, Devaux B et al. (1999) Cell Activation and Apoptosis by Bacterial Lipoproteins Through Toll-like Receptor-2. Science 285: 736-739. doi:. PubMed: 10426996. doi: 10.1126/science.285.5428.736.
67. Underhill DM, Ozinsky A, Smith KD, Aderem A (1999) Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci U S A 96: 14459-14463. doi:. PubMed: 10588727. doi: 10.1073/pnas.96.25.14459.
68. Gao L-Y, Abu Kwaik Y (1999) Activation of Caspase 3 during Legionella pneumophila-Induced Apoptosis. Infection and Immunity 67: 4886-4894.
CrossRef
PubMed/NCBI
Google Scholar
69. Seipp S, Schmich J, Leitz T (2001) Apoptosis – a death-inducing mechanism tightly linked with morphogenesis in Hydractina echinata (Cnidaria, Hydrozoa). Development 128: 4891-4898. PubMed: 11731468.
70. Dunn SR, Weis VM (2009) Apoptosis as a post-phagocytic winnowing mechanism in a coral-dinoflagellate mutualism. Environ Microbiol 11: 268-276. doi:. PubMed: 19125818. doi: 10.1111/j.1462-2920.2008.01774.x.
71. Richier S, Sabourault C, Courtiade J, Zucchini N, Allemand D et al. (2006) Oxidative stress and apoptotic events during thermal stress in the symbiotic sea anemone, Anemonia viridis. FEBS J 273: 4186-4198. doi:. PubMed: 16907933. doi: 10.1111/j.1742-4658.2006.05414.x.
72. Dunn SR, Schnitzler CE, Weis VM (2007) Apoptosis and autophagy as mechanisms of dinoflagellate symbiont release during cnidarian bleaching: every which way you lose. Proc Biol Sci 274: 3079-3085. doi:. PubMed: 17925275. doi: 10.1098/rspb.2007.0711.
73. Pernice M, Dunn SR, Miard T, Dufour S, Dove S et al. (2011) Regulation of Apoptotic Mediators Reveals Dynamic Responses to Thermal Stress in the Reef Building Coral Acropora millepora. PLOS ONE 6: e16095. doi:. PubMed: 21283671. doi: 10.1371/journal.pone.0016095.
74. Ainsworth TD, Kvennefors EC, Blackall LL, Fine M, Hoegh-Guldberg O (2007) Disease and cell death in white syndrome of Acroporid corals on the Great Barrier Reef. Marine Biology 151: 19-29. doi:. doi: 10.1007/s00227-006-0449-3.
75. Samuelsson B (1991) Arachidonic acid metabolism: role in inflammation. Zeitschrift fur Rheumatologie 50 (Suppl 1): 3-6. PubMed: 1907059.
76. Waite M, DeChatelet LR, King L, Shirley PS (1979) Phagocytosis-induced release of arachidonic acid from human neutrophils. Biochem Biophys Res Commun 90: 984-992. doi:. PubMed: 508358. doi: 10.1016/0006-291x(79)91924-7.
77. Penzo D, Petronilli V, Angelin A, Cusan C, Colonna R et al. (2004) Arachidonic Acid Released by Phospholipase A2 Activation Triggers Ca2+-dependent Apoptosis through the Mitochondrial Pathway. J Biol Chem 279: 25219-25225. doi:. PubMed: 15070903. doi: 10.1074/jbc.m310381200.
78. Brash AR, Baertschi SW, Ingram CD, Harris TM (1987) On non-cyclooxygenase prostaglandin synthesis in the sea whip coral, Plexaura homomalla: an 8(R)-lipoxygenase pathway leads to formation of an alpha-ketol and a Racemic prostanoid. J Biol Chem 262: 15829-15839. PubMed: 2824470.
79. Corey EJ, D’Alarcao M, Matsuda SPT, Lansbury PT, Yamada Y (1987) Intermediacy of 8-(R)-HPETE in the conversion of arachidonic acid to pre-clavulone a by Clavularia viridis. Implications for the biosynthesis of marine prostanoids. Journal of the American Chemical Society 109: 289-290. doi:. doi: 10.1021/ja00235a053.
80. Corey EJ, Matsuda SPT, Nagata R, Cleaver MB (1988) Biosynthesis of 8-R-HPETE and preclavulone-A from arachidonate in several species of caribbean coral. A widespread route to marine prostanoids. Tetrahedron Letters 29: 2555-2558. doi:. doi: 10.1016/s0040-4039(00)86110-9.
81. Koljak R, Boutaud O, Shieh B-H, Samel N, Brash AR (1997) Identification of a Naturally Occurring Peroxidase-Lipoxygenase Fusion Protein. Science 277: 1994-1996. doi:. PubMed: 9302294. doi: 10.1126/science.277.5334.1994.
82. Weis VM (2008) Cellular mechanisms of Cnidarian bleaching: stress causes the collapse of symbiosis. J Exp Biol 211: 3059-3066. doi:. PubMed: 18805804. doi: 10.1242/jeb.009597.
83. Tijet N, Brash AR (2002) Allene oxide synthases and allene oxides. Prostaglandins & Other Lipid Mediators 68-69: 423-431. doi: 10.1016/s0090-6980(02)00046-1.
CrossRef
PubMed/NCBI
Google Scholar
84. Varvas K, Järving I, Koljak R, Valmsen K, Brash AR et al. (1999) Evidence of a Cyclooxygenase-related Prostaglandin Synthesis in Coral. J Biol Chem 274: 9923-9929. doi:. PubMed: 10187766. doi: 10.1074/jbc.274.15.9923.
85. Weis VM, Allemand D (2009) Physiology. What determines coral health? Science 324: 1153-1155. doi:. PubMed: 19478172. doi: 10.1126/science.1172540.
86. Glauser G, Grata E, Dubugnon L, Rudaz S, Farmer EE et al. (2008) Spatial and Temporal Dynamics of Jasmonate Synthesis and Accumulation in Arabidopsis in Response to Wounding. J Biol Chem 283: 16400-16407. doi:. PubMed: 18400744. doi: 10.1074/jbc.m801760200.
87. Ziegler J, Keinänen M, Baldwin IT (2001) Herbivore-induced allene oxide synthase transcripts and jasmonic acid in Nicotiana attenuata. Phytochemistry 58: 729-738. doi:. PubMed: 11672737. doi: 10.1016/s0031-9422(01)00284-9.
88. Polato NR, Voolstra CR, Schnetzer J, DeSalvo MK, Randall CJ et al. (2010) Location-Specific Responses to Thermal Stress in Larvae of the Reef-Building Coral Montastraea faveolata. PLOS ONE 5: e11221. doi:. PubMed: 20585643. doi: 10.1371/journal.pone.0011221.
89. DeSalvo MK, Voolstra CR, Sunagawa S, Schwarz JA, Stillman JH et al. (2008) Differential gene expression during thermal stress and bleaching in the Caribbean coral Montastraea faveolata. Mol Ecol 17: 3952-3971. doi:. PubMed: 18662230. doi: 10.1111/j.1365-294x.2008.03879.x.
90. Cailhier J-F, Sirois I, Laplante P, Lepage S, Raymond M-A et al. (2008) Caspase-3 Activation Triggers Extracellular Cathepsin L Release and Endorepellin Proteolysis. J Biol Chem 283: 27220-27229. doi:. PubMed: 18658137. doi: 10.1074/jbc.m801164200.
91. Gilgès D, Vinit MA, Callebaut I, Coulombel L, Cacheux V et al. (2000) Polydom: a secreted protein with pentraxin, complement control protein, epidermal growth factor and von Willebrand factor A domains. Biochem J 352: 49-59. doi:. PubMed: 11062057. doi: 10.1042/0264-6021:3520049.
92. Schwarz RS, Bosch TC, Cadavid LF (2008) Evolution of polydom-like molecules: identification and characterization of cnidarian polydom (Cnpolydom) in the basal metazoan Hydractinia. Dev Comp Immunol 32: 1192-1210. doi:. PubMed: 18466971. doi: 10.1016/j.dci.2008.03.007.
93. Wiens M, Korzhev M, Krasko A, Thakur NL, Perović-Ottstadt S et al. (2005) Innate immune defense of the sponge Suberites domuncula against bacteria involves a MyD88-dependent signaling pathway. Induction of a perforin-like molecule. J Biol Chem 280: 27949-27959. doi:. PubMed: 15923643. doi: 10.1074/jbc.m504049200.
94. Leggat W, Seneca F, Wasmund K, Ukani L, Yellowlees D et al. (2011) Differential Responses of the Coral Host and Their Algal Symbiont to Thermal Stress. PLOS ONE 6: e26687. doi:. doi: 10.1371/journal.pone.0026687.
95. Craig DW, Pearson JV, Szelinger S, Sekar A, Redman M, et al. (2008) Identification of genetic variants using bar-coded multiplexed sequencing. Nat Methods 5(10): 887-893. doi:. PubMed: 18794863. doi: 10.1038/nmeth.1251.
96. Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome.
97. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215: 403-410. doi:. PubMed: 2231712. doi: 10.1016/s0022-2836(05)80360-2.
98. Consortium U (2012). Reorganizing the Protein Space at The Universal Protein Resource (UniProt). doi: 10.1093/nar/gkr981.




