http://www.oceanfdn.org/blog/?p=1159
by Mark J. Spalding, President of The Ocean Foundation
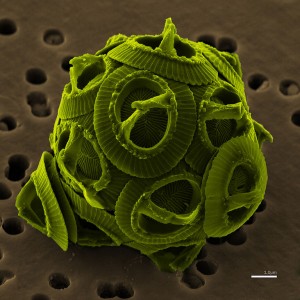
A magnified image of the coccolithophore, Gephyrocapsa oceanica Kamptner. Coccolithophores are single-celled algae, protists, and phytoplankton and considered especially vulnerable to ocean acidification due to their calcium carbonate shells. (Image: Gephyrocapsa oceanica Kamptner from Mie Prefecture, Japan. SEM:JEOL JSM-6330F. Scale bar = 1.0 micron. Licensed under the Creative Commons Attribution-Share Alike 2.5 Generic license.)
Last week I was in Monterey, California for the 3rd International Symposium on the Ocean in a High CO2 World, which was simultaneous to the BLUE Ocean Film Festival at the hotel next door (but that is a whole other story to tell). At the symposium, I joined hundreds of other attendees in learning about the current state of knowledge and potential solutions to address the effects of elevated carbon dioxide (CO2) on the health of our oceans and the life within. We call the consequences ocean acidification because the pH of our oceans is getting lower and thus more acidic, with significant potential harm to ocean systems as we know them.
OCEAN ACIDIFICATION
The 2012 High CO2 meeting was a huge leap from the 2nd meeting in Monaco in 2008. Over 500 attendees and 146 speakers, representing 37 nations, were gathered to discuss the issues at hand. It included a first major inclusion of socio-economic studies. And, while the primary focus was still on marine life organism responses to ocean acidification and what that means for ocean system, everyone was in agreement that our knowledge about effects and potential solutions has greatly advanced in the last four years.
For my part, I sat in rapt amazement as one scientist after another gave a history of the science around ocean acidification (OA), information on the current state of science knowledge about OA, and our first inklings of specifics about the ecosystem and economic consequences of a warmer ocean that is more acidic and has lower oxygen levels.
As Dr. Sam Dupont of The Sven Lovén Centre for Marine Sciences – Kristineberg, Sweden said:
What do we know?
Ocean Acidification is real
It is directly coming from our carbon emissions
It is happening fast
Impact is certain
Extinctions are certain
It is already visible in the systems
Change will happen
Hot, sour and breathless are all symptoms of the same disease.
Especially when combined with other diseases, OA becomes a major threat.
We can expect lots of variability, as well as positive and negative carry over effects.
Some species will alter behavior under OA.
We know enough to act
We know a major catastrophic event is coming
We know how to prevent it
We know what we don’t know
We know what we need to do (in science)
We know what we will focus on (bringing solutions)
But, we should be prepared for surprises; we have so completely perturbed the system.
Dr. Dupont closed his comments with a photo of his two children with a powerful and striking two sentence statement:
I am not an activist, I am a scientist. But, I am also a responsible father.
The first clear statement that CO2 accumulation in the sea could have “possible catastrophic biological consequences” was published in 1974 (Whitfield, M. 1974. Accumulation of fossil CO2 in the atmosphere and in the sea. Nature 247:523-525.). Four years later, in 1978, the direct linkage of fossil fuels to CO2 detection in the ocean was established. Between 1974 and 1980, numerous studies began to demonstrate the actual change in ocean alkalinity. And, finally, in 2004, the spectre of ocean acidification (OA) became accepted by the scientific community at large, and the first of the high CO2 symposia were held.
The following spring, the marine funders were briefed at their annual meeting in Monterey, including a field trip to see some cutting edge research at Monterey Bay Aquarium Research Institute (MBARI). I should note that most of us had to be reminded of what the pH scale means, although everyone seemed to recollect using the litmus paper to test liquids in middle school science classrooms. Fortunately, the experts were willing to explain that the pH scale is from 0 to 14, with 7 being neutral. The lower the pH, means lower alkalinity, or more acidity.
At this point, it has become clear that the early interest in ocean pH has produced some concrete results. We have some credible scientific studies, which tell us that as ocean pH falls, some species will thrive, some survive, some are replaced, and many go extinct (the expected result is loss of biodiversity, but a maintenance of biomass). This broad conclusion is the result of lab experiments, field exposure experiments, observations at naturally high CO2 locations, and studies focused on fossil records from previous OA events in history.
WHAT WE KNOW FROM PAST OCEAN ACIDIFICATION EVENTS
While we can see changes in ocean chemistry and ocean sea surface temperature over the 200 some years since the industrial revolution, we need to go back further in time for a control comparison (but not too far back). So the Pre-Cambrian period (the first 7/8s of Earth’s geological history) has been identified as the only good geological analog (if for no other reason than similar species) and includes some periods with lower pH. These previous periods experienced a similar high CO2 world with lower pH, lower oxygen levels, and warmer sea surface temperatures.
However, there is nothing in the historical record that equals our current rate of change of pH or temperature.
The last dramatic ocean acidification event is known as PETM, or the Paleocene–Eocene Thermal Maximum, which took place 55 million years ago and is our best comparison. It happened rapidly (over about 2,000 years) it lasted for 50,000 years. We have strong data/evidence for it – and thus scientists use it as our best available analog for a massive carbon release.
However, it is not a perfect analog. We measure these releases in petagrams. PgC are Petagrams of carbon: 1 petagram = 1015 grams = 1 billion metric tons. The PETM represents a period when 3,000 PgC were released over a few thousand years. What matters is the rate of change in the last 270 years (the industrial revolution), as we have pumped 5,000 PgC of carbon into our planet’s atmosphere. This means the release then was 1 PgC y-1 compared to the industrial revolution, which is 9 PgC y-1. Or, if you are just an international law guy like me, this translates to the stark reality that what we have done in just under three centuries is 10 times worse than what caused the extinction events in the ocean at PETM.
The PETM ocean acidification event caused big changes in the global ocean systems, including some extinctions. Interestingly, the science indicates that total biomass stayed about even, with dinoflagellate blooms and similar events offsetting the loss of other species. In total, the geological record shows a wide range of consequences: blooms, extinctions, turnovers, calcification changes, and dwarfism. Thus, OA causes a significant biotic reaction even when the rate of change is much slower than our current rate of carbon emissions. But, because it was much slower, the “future is uncharted territory in the evolutionary history of most modern organisms.”
Thus, this anthropogenic OA event will easily top PETM in impact. AND, we should expect to see changes in how change occurs because of we have so disturbed the system. Translation: Expect to be surprised.
ECOSYSTEM AND SPECIES RESPONSE
Brilliant shades of blue and green explode across the Barents Sea just north of the Scandinavian peninsula in this natural-color image, created by a massive bloom of phytoplankton that are common in the area each August. The milky blue color strongly suggests that the bloom contains coccolithophores, microscopic plankton that are plated with white calcium carbonate. When viewed through ocean water, a coccolithophore bloom tends to be bright blue. The species is most likely Emiliana huxleyi, whose blooms tend to be triggered by high light levels during the 24-hour sunlight of Arctic summer. Ocean acidifications impact on plankton species is of particular concern given the potential to undermine the base of the ocean food chain. (Image: NASA Earth Observatory)
Brilliant shades of blue and green explode across the Barents Sea just north of the Scandinavian peninsula in this natural-color image, created by a massive bloom of phytoplankton that are common in the area each August. The milky blue color strongly suggests that the bloom contains coccolithophores, microscopic plankton that are plated with white calcium carbonate. When viewed through ocean water, a coccolithophore bloom tends to be bright blue. The species is most likely Emiliana huxleyi, whose blooms tend to be triggered by high light levels during the 24-hour sunlight of Arctic summer. Ocean acidifications impact on plankton species is of particular concern given the potential to undermine the base of the ocean food chain. (Image: NASA Earth Observatory)
Ocean acidification and temperature change both have carbon dioxide (CO2) as a driver. And, while they can interact, they are not running in parallel. Changes in pH are more linear, with smaller deviations, and are more homogenous in different geographical spaces. Temperature is far more variable, with wide deviations, and is substantially variable spatially.
Temperature is the dominant driver of change in the ocean. Thus, it is not a surprise that change is causing a shift in distribution of species to the extent they can adapt. And we have to remember that all species have limits to acclimation capacity. Of course, some species remain more sensitive than others because they have narrower boundaries of temperature in which they thrive. And, like other stressors, temperature extremes increase sensitivity to the effects of high CO2.
The pathway looks like this:
CO2 emissions → OA → biophysical impact → loss of ecosystem services (e.g. a reef dies, and no longer stops storm surges) → socio-economic impact (when the storm surge takes out the town pier)
Noting at the same time, that demand for ecosystem services is rising with population growth and increasing income (wealth)
To look at the effects, scientists have examined various mitigation scenarios (different rates of pH change) compared to maintaining the status quo which risks:
Simplification of diversity (up to 40%), and thus a reduction of ecosystem quality
There is little or no impact on abundance
Average size of various species decreases by 50%
OA causes shift away from dominance by calcifiers (organisms whose structure is formed of calcium-based material):
No hope for survival of corals which are utterly dependent on water at a certain pH to survive (and for cold water corals, warmer temperatures will exacerbate the problem);
Gastropods (thin-shelled sea snails) are the most sensitive of the mollusks;
There is a big impact on exoskeleton-bearing aquatic invertebrates, including various species of mollusks, crustaceans, and echinoderms (think clams, lobsters and urchins)
Within this category of species, arthropods (such as shrimp) are not as bad off, but there is a clear signal of their decline
Other invertebrates adapt faster (such as jellyfish or worms)
Fish, not so much, and fish may also have no place to migrate to (for example in SE Australia)
Some success for marine plants that may thrive on consuming CO2
Some evolution can occur on relatively short time scales, which may mean hope
Evolutionary rescue by less sensitive species or populations within species from standing genetic variation for pH tolerance (we can see this from breeding experiments; or from new mutations (which are rare))
So, the key question remains: Which species will be affected by OA? We have a good idea of the answer: bivalves, crustaceans, predators of calcifiers, and top predators in general. It is not difficult to envision how severe the financial consequences will be for the shellfish, seafood, and dive tourism industries alone, much less others in the network of suppliers and service. And in the face of the enormity of the problem, it can be hard to focus on solutions.
WHAT OUR RESPONSE SHOULD BE
Rising CO2 is the root cause (of the disease) [but like smoking, getting the smoker to quit is very hard]
We must treat the symptoms [high blood pressure, emphysema]
We must reduce other stressors [cut back on drinking and over-eating]
Reducing the sources of ocean acidification requires sustained source reduction efforts at both the global and the local scale. Global carbon dioxide emissions are the biggest driver of ocean acidification at the scale of the world’s ocean, so we must reduce them. Local additions of nitrogen and carbon from point sources, nonpoint sources, and natural sources can exacerbate the effects of ocean acidification by creating conditions that further accelerate pH reductions. Deposition of local air pollution (specifically carbon dioxide, nitrogen and sulfur oxide) can also contribute to reduced pH and acidification. Local action can help slow the pace of acidification. So, we need to quantify key anthropogenic and natural processes contributing to acidification.
The following are priority, near-term action items for addressing ocean acidification.
Quickly and significantly reduce global emissions of carbon dioxide to mitigate and reverse the acidification of our oceans.
Limit nutrient discharges entering marine waters from small and large on-site sewage systems, municipal wastewater facilities, and agriculture, thus limiting the stressors on ocean life to support adaptation and survival.
Implement effective clean water monitoring and best management practices, as well as revise existing and/or adopt new water quality standards to make them relevant to ocean acidification.
Investigate selective breeding for ocean acidification tolerance in shellfish and other vulnerable marine species.
Identify, monitor and manage the marine waters and species in potential refuges from ocean acidification so they may endure concurrent stresses.
Understand the association between water chemistry variables and shellfish production and survival in hatcheries and in the natural environment, promoting collaborations between scientists, managers, and shellfish growers. And, establish an emergency warning and response capacity when monitoring indicates a spike in low pH water that threatens sensitive habitat or shellfish industry operations.
Restore seagrass, mangroves, marsh grass etc. that will take up and fix dissolved carbon in marine waters and locally prevent (or slow) changes in the pH of those marine waters
Educate the public about the problem of ocean acidification and its consequences for marine ecosystems, economy, and cultures
The good news is that progress is being made on all of these fronts. Globally, tens of thousands of people are working to reduce greenhouse gas emissions (including CO2) at the international, national and local levels (Item 1). And, in the USA, item 8 is the primary focus of a coalition of NGOs coordinated by our friends at Ocean Conservancy. For item 7, TOF hosts our own effort to restore damaged seagrass meadows. But, in an exciting development for items 2-7, we are working with key state decision-makers in four coastal states to develop, share and introduce legislation designed to address OA. The existing effects of ocean acidification on shellfish and other marine life in Washington and Oregon’s coastal waters have inspired action in a number of ways.
All of the speakers at the conference made it clear that more information is needed—especially about where pH is changing rapidly, which species will be able to thrive, survive, or adapt, and local and regional strategies that are working. At the same time, the takeaway lesson was that even though we do not know everything we want to know about ocean acidification, we can and should be taking steps to mitigate its effects. We will continue to work with our donors, advisors, and other members of the TOF community to support the solutions.
Special thanks to Mark Spalding, The Ocean Foundation